REACH re-authorisation focus will be on your substitution activities
Your REACH re-authorisation application will be all about substitution!
Do you need to re-apply for REACH authorisation to continue use of an authorization listed substance?
If yes, you will need to carefully consider your re-application strategy well in advance of the expiry date for your current authorization! Since you last submitted, the process has evolved and you will need to describe your substitution efforts, past, present and future!
Re-application process in brief
The re-application process is very similar to the initial application in terms of process and deadlines. The re-application to ECHA must be submitted 18 months before the expiry of the authorisation period to benefit from “transitional arrangements” (i.e. the right to continue use after the expiry date even if the Commission has not issued a decision granting your re-authorisation).
Full details are available on the ECHA website.
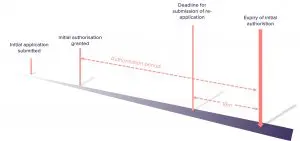
What do you need to submit with your re-application?
You will need to submit updated versions of all your initial application reports to describe your current situation in terms of substitution and demonstrate your compliance with any conditions, monitoring arrangements, and any other requirements imposed in your decision.
Due to court challenges on granted authorisations, there is now a requirement to submit either a substitution plan or an R&D plan depending on whether it is considered that there are “suitable alternatives generally available” (SAGA) for your specific use. You may not have submitted either with your initial application. However you will still need to describe the actions you have taken to substitute during your current authorisation period and submit an updated plan that describes the actions you intend to take to substitute.
It’s all about substitution!
Your substitution / R&D plan will be critical for your successful re-application. The key assessment criterion is credibility –you must demonstrate that you have implemented the previous plan (if submitted) or sufficiently justify why you couldn’t implement it and ensure that your updated plan is sufficiently detailed.
This means that you will need to document all the actions you have taken to either identify alternatives or take a suitable alternative into implementation during the authorisation period. You will need to carefully consider R&D plan or substitution plan submitted with your initial application and describe any deviations taken and contingency measures put in place.
Apeiron-team can assist you with your authorization reapplication by:
- Making sure your chemical safety report (CSR) is up to date
- Updating or development of your analysis of alternatives (AoA)
- Development of your socio-economic analysis (SEA)
- Development of your substitution/ R&D plan.
Demonstrating compliance with all monitoring arrangements and conditions imposed in your current authorisation decision!
Your current authorisation decision may have imposed requirements to put emissions and occupational exposure monitoring programs in place with annual monitoring campaigns. There may also be a requirement for an annual review of your operating conditions and risk management measures with reference to the results of the annual monitoring campaign. All monitoring data and associated documentation relating to the annual review of the data will need to be submitted with your re-application.
Apeiron-team can assist you with determining the actions you need to take to be able to demonstrate compliance with imposed conditions and monitoring arrangements. We can assist you with your monitoring campaigns and assist you with your review of the operating conditions and risk management measures.
Reach out to our experts
Our authorisation team can assist you with every step in preparing for your re-application!
Contact our experts for more information on full range of authorisation and restriction services.
Like the sound of Apeiron?
We'd love to know more about you! We are always looking for people who are driven by passion.




